Released 18:29:00 09 December 2024
Blenrep shows significant overall survival benefit, reducing the risk of death by 42% in multiple myeloma at or after first relapse
- DREAMM-7 trial shows sustained overall survival benefit for Blenrep (belantamab mafodotin) combination versus daratumumab combination; benefit seen early and maintained through follow-up.
- Data build on findings from DREAMM-7 and DREAMM-8 and support the potential for Blenrep combinations to become standard of care.
- Blenrep combinations are under regulatory review in seven major markets.
GSK plc (LSE/NYSE: GSK) today announced statistically significant and clinically meaningful overall survival (OS) results from a planned interim analysis of the DREAMM-7 trial evaluating Blenrep (belantamab mafodotin) in combination with bortezomib plus dexamethasone (BVd) versus daratumumab in combination with bortezomib plus dexamethasone (DVd) as a second line or later treatment for relapsed or refractory multiple myeloma. These data were featured today in an oral presentation at the 66th American Society of Hematology (ASH) Annual Meeting and Exposition.
The OS findings from DREAMM-7 build on previous data from the DREAMM-7[1] and DREAMM-8[2] trials, which showed a statistically significant and clinically meaningful improvement in progression-free survival (PFS) for both belantamab mafodotin-based combinations versus standard of care comparators.
Hesham Abdullah, Senior Vice President, Global Head Oncology, R&D, GSK, said: “The compelling overall survival data from the DREAMM-7 trial establish the potential of Blenrep in combination to significantly extend the lives of patients with multiple myeloma at or after first relapse. This represents an important advancement that could redefine the treatment of relapsed or refractory multiple myeloma.”
With a median follow up of 39.4 months, the analysis presented today shows a statistically significant 42% reduction in the risk of death among patients receiving the belantamab mafodotin combination (n=243) versus the daratumumab-based comparator (n=251) (HR 0.58; 95% CI: 0.43-0.79; p=0.00023). Although the median overall survival (mOS) was not reached in either arm of the study, the projected mOS for BVd is 84 months compared to 51 months for DVd.[3]
The three-year OS rate was 74% in the belantamab mafodotin combination arm and 60% in the daratumumab combination arm. The survival benefit favouring BVd was seen as early as four months and was sustained over time as illustrated by the separation of the lines in the Kaplan-Meier curve shown here.
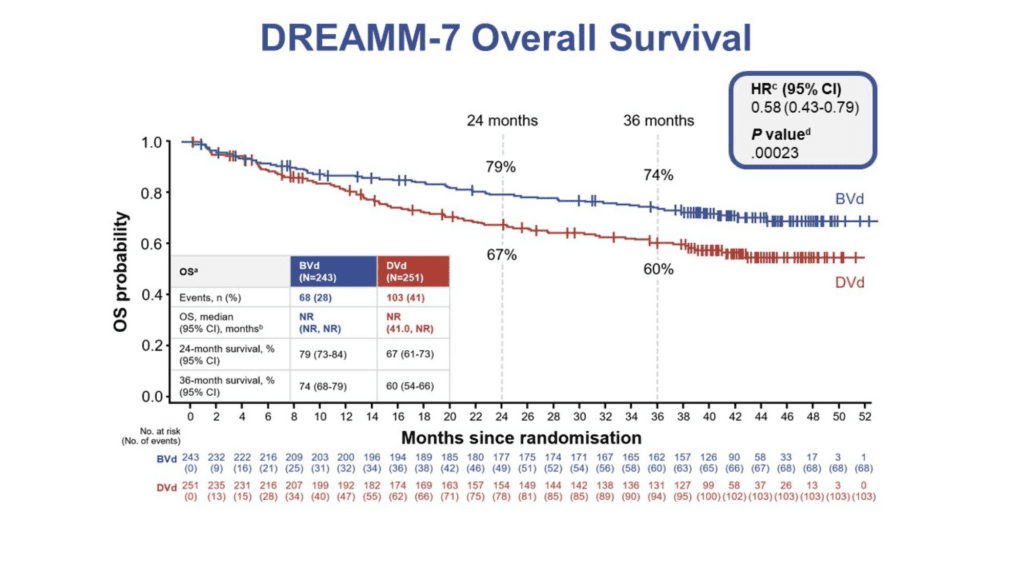
BVd, belantamab mafodotin, bortezomib, and dexamethasone; DVd, daratumumab, bortezomib, and dexamethasone; HR, hazard ratio; ITT, intention to treat; NR, not reached; OS, overall survival; R-ISS, Revised International Staging System.
a Two patients in the ITT population were randomised, not treated, rescreened, and rerandomised. They are counted as 4 unique patients in this output.
b CIs were estimated using the Brookmeyer-Crowley method.
c HRs were estimated using a Cox proportional hazards model stratified by the number of lines of prior therapy (1 vs 2 or 3 vs ≥4), prior bortezomib (no vs yes), and R-ISS stage at screening (I vs II or III), with a covariate of treatment.
d P value is from a 1-sided stratified log-rank test. At 171 actual events (48.2% OS information fraction), OS was declared significant if the P value was <.00112.
MarÃa-Victoria Mateos, MD, PhD, Head of Myeloma and Clinical Trials Unit, Haematology Department and Professor of Medicine at the University of Salamanca, Spain, and DREAMM-7 principal investigator, said: “The totality of evidence from DREAMM-7 represents a potential paradigm shift for multiple myeloma patients who have experienced a relapse or become refractory to initial treatment. The OS results shown with the belantamab mafodotin combination in DREAMM-7 further cement the potential of this regimen to prolong the lives of patients with relapsed or refractory multiple myeloma compared to a standard of care daratumumab combination.”
The belantamab mafodotin combination also showed statistically significant superiority on the key secondary endpoint of minimal residual disease (MRD) negativity (no detectable cancer cells) compared to the daratumumab combination. The greater than 2.5-fold improvement in the rate of MRD negativity seen at the time of the primary analysis for patients who received BVd can now be declared as statistically significant (p<0.00001) after the positive OS readout based on the predefined testing procedure. This further underscores the transformative potential of this belantamab mafodotin combination for multiple myeloma patients at or after their first relapse.
In addition to OS and MRD negativity, the belantamab mafodotin combination resulted in clinically meaningful improvements in all key secondary efficacy endpoints compared to the daratumumab combination, including duration of response (DOR) and progression-free survival 2 (PFS 2). The results indicate deeper and more durable responses among patients treated with BVd compared to DVd.
The safety and tolerability of the belantamab mafodotin regimen were consistent with the primary analysis and known safety profile of the individual agents. Grade 3 or higher adverse events of clinical interest in the belantamab mafodotin combination and daratumumab combination arms, respectively included thrombocytopenia (56% versus 35%; 34 versus 25 patients/100 person-years); anaemia (9% versus 10%; exposure-adjusted rate [per 100 person-years] not reported); and neutropenia (14% versus 10%; 8 versus 7 patients/100 person-years).
Eye-related side effects, a known risk of treatment with belantamab mafodotin, were generally manageable and resolvable with dose modification, and led to a low (10%) treatment discontinuation rate.
Full data summaries for OS and other key secondary endpoints are shown below.
| Key Secondary Endpoints | ||
Endpoint | belantamab mafodotin + bortezomib + dexamethasone (BVd) n=243 | daratumumab + bortezomib + dexamethasone (DVd) n=251 |
| OS (overall survival), HR (95% CI) | 0.58 (0.43-0.79) | |
| P-value1 | p=0.00023 | |
| OS, median (95% CI), months | NR (NR-NR) | NR (41.0-NR) |
| OS rate at 24 months, % (95% CI) | 79% (73-84) | 67% (61-73) |
| OS rate at 36 months, % (95% CI) | 74% (68-79) | 60% (54-66) |
| MRD (minimal residual disease) negativity rate for patients with CR or better, % (95% CI) | 25.1% ​(19.8-31.0)​ | 10.4% ​(6.9-14.8)​ |
| ORR (overall response rate), % (95% CI) | 83.1% ​(77.8-87.6) | 71.3% ​(65.3-76.8)​ |
| CR (complete response), or better, % (95% CI) | 35.8% ​(29.8-42.2) | 17.5% ​(13.0-22.8)​ |
| VGPR (very good partial response), or better, % (95% CI) | 66.3%​ (59.9-72.2) | 46.2% ​(39.9-52.6)​ |
| Median DOR (duration of response) (95% CI), months | 40.8 (30.5-NR)​ | 17.8 (13.8-23.6)​ |
| Median PFS 2 (progression-free survival 2), months | NR (45.6-NR)​ | 33.4 (26.7-44.9) |
| HR | 0.59 (0.45-0.77) | |
1One-sided p-value based on stratified log-rank test.
In 2024, regulatory filings for belantamab mafodotin combinations for the treatment of relapsed or refractory multiple myeloma based on the results of the DREAMM-7 and DREAMM-8 trials have been accepted in the US[4], European Union[5], Japan[6] (with priority review), China (for DREAMM-7 only, with priority review; Breakthrough Therapy Designation[7] also granted), United Kingdom, Canada and Switzerland (with priority review for DREAMM-8).
References
- GSK press release issued 05 February 2024. DREAMM-7 phase III trial shows Blenrep combination nearly tripled median progression-free survival versus standard of care combination in patients with relapsed/refractory multiple myeloma. Available at: https://www.gsk.com/en-gb/media/press-releases/dreamm-7-phase-iii-trial-shows-pfs-improvement-and-strong-os-trend-for-blenrep-combo-versus-soc-combo-in-multiple-myeloma/.
- GSK press release issued 02 June 2024. Blenrep combination reduced the risk of disease progression or death by nearly 50% versus standard of care combination in relapsed/refractory multiple myeloma. Available at: https://www.gsk.com/en-gb/media/press-releases/blenrep-combination-reduced-the-risk-of-disease-progression/.
- Post hoc analysis using simulation to predict median OS values in each arm utilising the observed data at the interim analysis with 39.4-month median follow up to extrapolate time to death of ongoing censored patients. Predicted median OS values subject to change as data matures.
- GSK press release issued 25 November 2024. Blenrep combinations accepted for review by the US FDA for the treatment of relapsed/refractory multiple myeloma. Available at: https://www.gsk.com/en-gb/media/press-releases/blenrep-combinations-accepted-for-review-by-the-us-fda-for-the-treatment-of-relapsedrefractory-multiple-myeloma/.
- GSK press release issued 19 July 2024. Blenrep (belantamab mafodotin) combinations in multiple myeloma accepted for review by the European Medicines Agency. Available at: https://www.gsk.com/en-gb/media/press-releases/blenrep-belantamab-mafodotin-combinations-in-multiple-myeloma-application-accepted-for-review-by-the-european-medicines-agency/.
- GSK press release issued 17 September 2024. Blenrep (belantamab mafodotin) combinations in relapsed/refractory multiple myeloma accepted for regulatory review in Japan. Available at: https://www.gsk.com/en-gb/media/press-releases/blenrep-belantamab-mafodotin-combinations-in-relapsedrefractory-multiple-myeloma-accepted-for-regulatory-review-in-japan/.
- GSK press release issued 13 September 2024. Blenrep (belantamab mafodotin) in combination receives Breakthrough Therapy Designation in China for treatment of relapsed/refractory multiple myeloma. Available at: https://www.gsk.com/en-gb/media/press-releases/blenrep-belantamab-mafodotin-in-combination-receives-breakthrough-therapy-designation-in-china-for-treatment-of-relapsedrefractory-multiple-myeloma/.